Egnyte for Life Sciences
Accelerate the Medical Writing Process
Create and disseminate content securely and easily to HCPs and KOLs with full visibility into the document lifecycle
Medical Writing
Simplify medical content creation with Egnyte's secure real-time co-editing platform. Enable medical writing teams to author, review, and collaborate on study documents in one unified environment. Ensure projects stay on track with built-in regulatory compliance, audit history tracking and complete file visibility.
Shorten the Document Creation Lifecycle
- Co-edit manuscripts and product documentation with native support for Office365 and Google Workspace; directly annotate PDFs
- View and export full audit trails
- Minimize turnaround time with customizable workflows
Securely Share Content with HCPs and KOLs
- Easily segregate data from multiple projects with granular folder and file-level permissions
- Customizable retention policies
- Ensure content adheres to 21 CFR Part 11 and ALCOA+ guidelines prior to submission
Structured Review and Approval
- Templatized workflows to support the entire document lifecycle
- Part-11 compliant e-signatures
- Grant external collaborators the ability to review and approve document changes
The Egnyte Solution
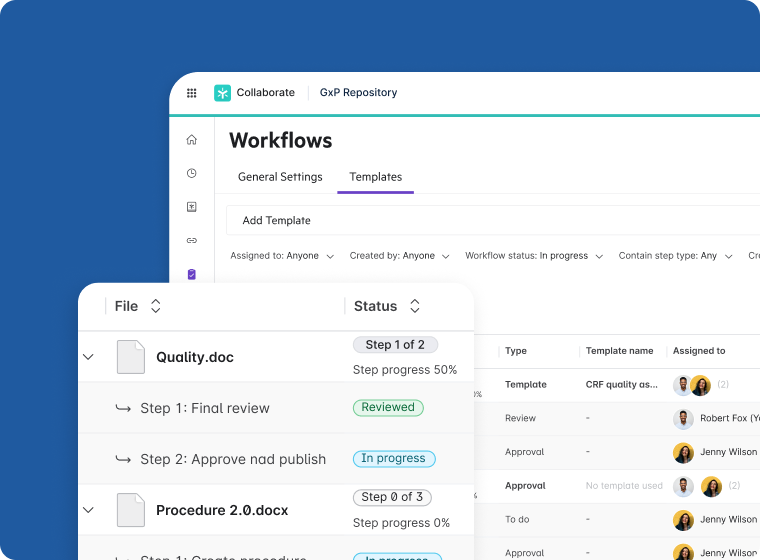
Document Lifecycle Management
- Accelerate review cycles with tools such as PDF annotation, version control, and workflow templates
- Manage regulatory documents and IP in a GxP-compliant environment
- Create and co-edit content securely across the biotech partner ecosystem
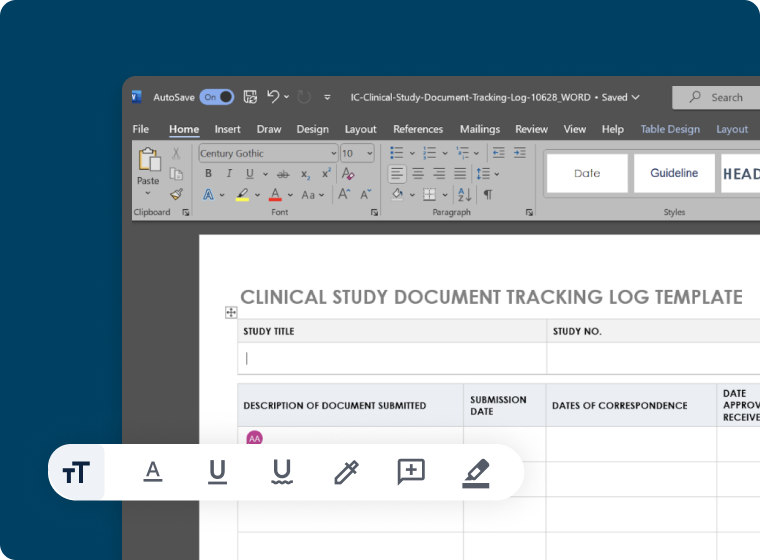
Secure Collaboration
- Built-in co-editing support for MS-Word and Google Docs
- Customizable templates to accelerate medical writing workflows
- Review content in real-time to adhere to drug development timelines
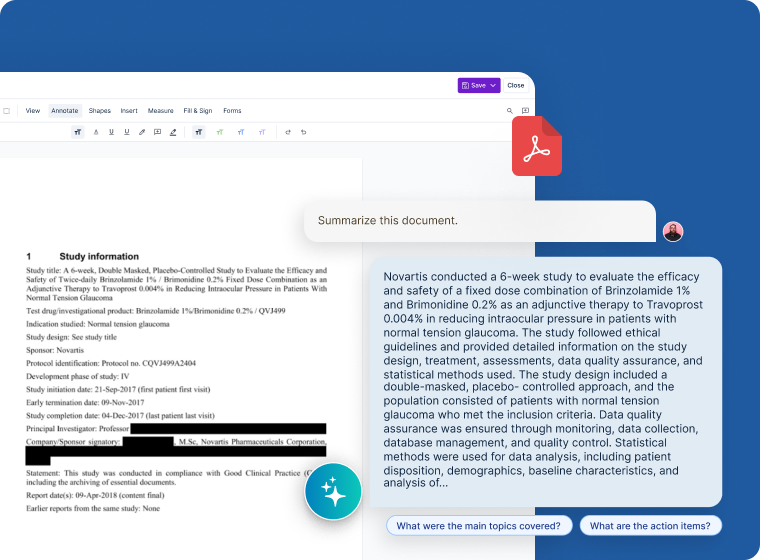
Unified Intelligent Platform
- Generative AI capabilities to summarize clinical and non-clinical content
- Seamlessly locate files, protocols, and scientific manuscripts in a secure, centralized repository
- Deploy simple-to-use governance controls and reporting for medical writing teams
