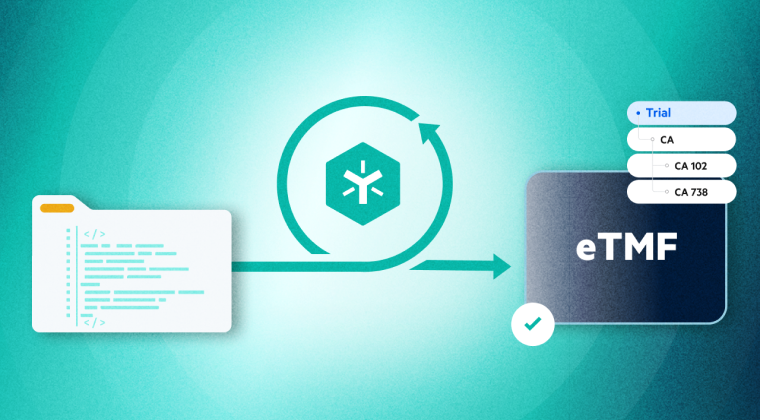
Accelerate Clinical Trials
Streamline clinical trial data management and maintain inspection readiness across trial sites and CROs.
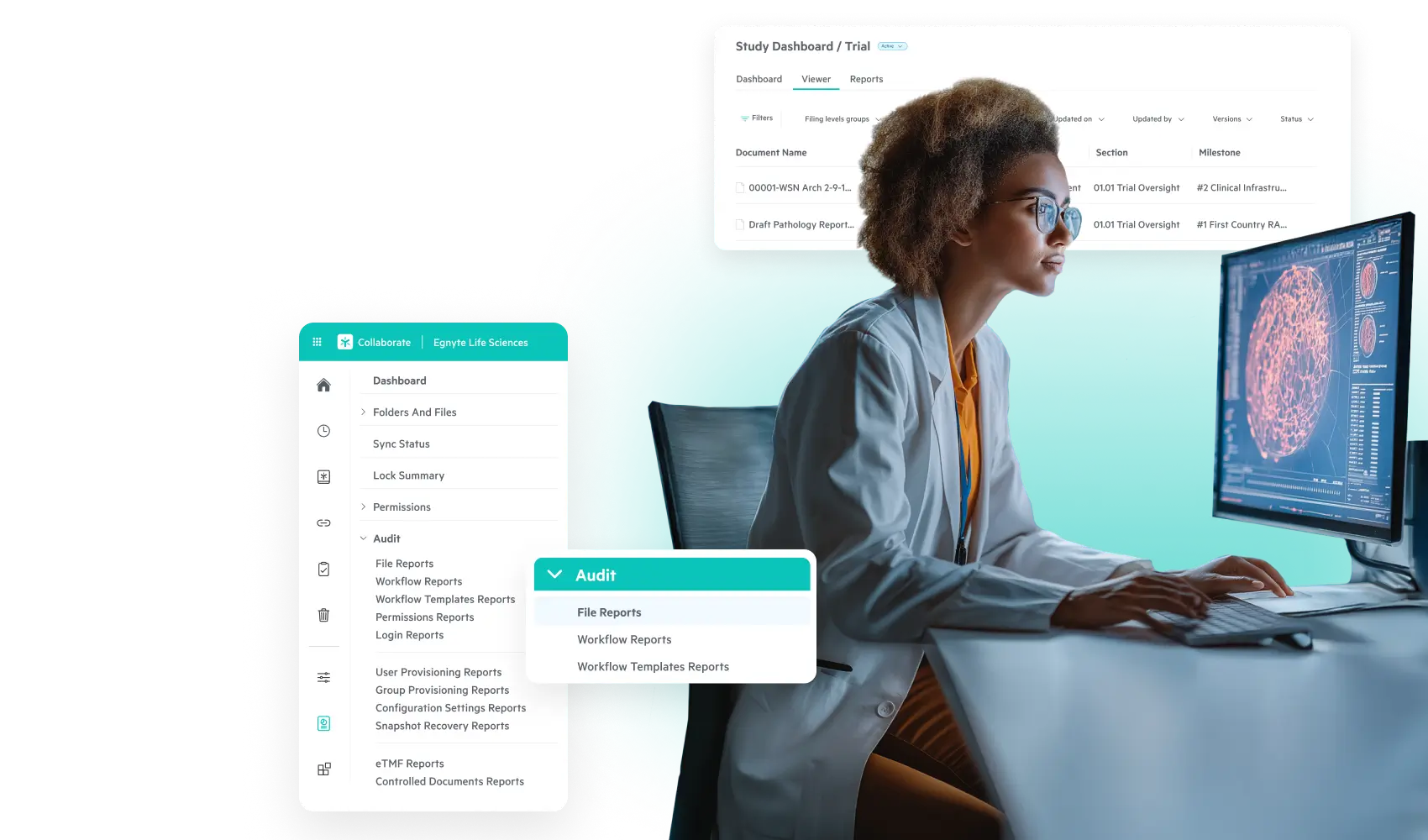
Streamline Trial Operations With
End-to-End Governance
Accelerate study startup and execution and maintain compliance with Egnyte’s
simplified solutions for trial data management.
simplified solutions for trial data management.
Simplify Clinical Trial Data: From eTMF Management to Analysis and Governance
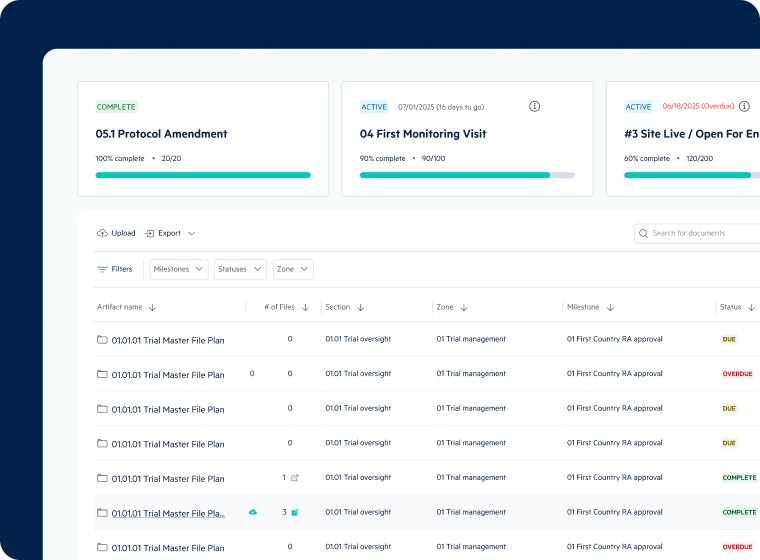
Inspection-Ready eTMF Management
- Automated eTMF workflows streamline document collection and filing
- Real-time tracking of essential document completeness and quality
- Integrated site and CRO collaboration within your eTMF
- Inspection-ready TMF with comprehensive audit trail monitoring

Fast and Accurate Statistical Data Analysis
- Secure validated storage environment for statistical analysis files
- Streamlined collaboration between biostatisticians and research teams
- Multilingual support for SAS, R, and Python
- Automated audit trails for statistical computing workflows
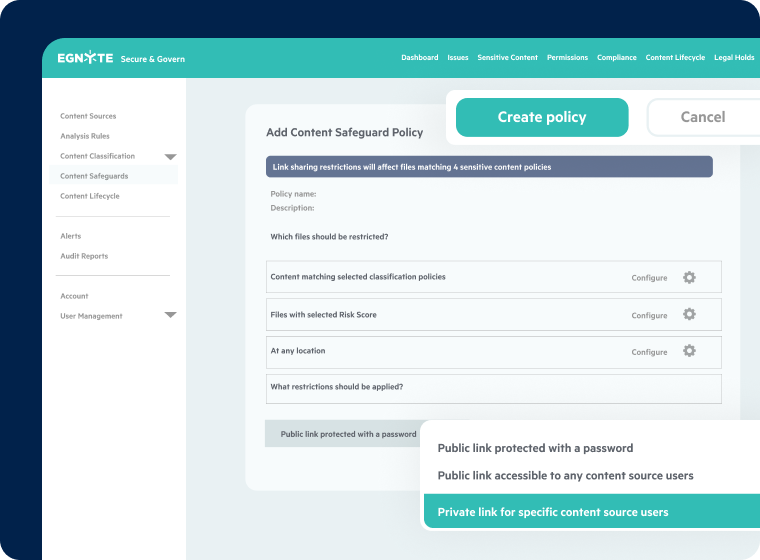
Comprehensive Data Governance
- Granular access controls for trial data
- Secure external collaboration tools
- End-to-end encryption
- Built-in GxP and ICH E6 (R3) compliance

Integration With Leading
Life Sciences Applications
Egnyte integrates seamlessly with your systems, making it easy to manage, collaborate on, and maintain compliance.