Maintain Regulatory
Compliance and Quality
Ensure compliance of controlled documents, automate
workflows, and streamline regulatory submissions with
Egnyte’s secure, GxP-compliant solution.
workflows, and streamline regulatory submissions with
Egnyte’s secure, GxP-compliant solution.

Simplify Quality and Regulatory Compliance
in Life Sciences With Egnyte
Egnyte’s intelligent platform centralizes document control, automates audit-ready workflows,
and safeguards sensitive data so you can achieve seamless compliance with industry regulations
like GxP and ICH E6 (R3) while maintaining high-quality standards across your organization.
and safeguards sensitive data so you can achieve seamless compliance with industry regulations
like GxP and ICH E6 (R3) while maintaining high-quality standards across your organization.
Elevate Compliance Readiness and Optimize Quality Management

Centralized Quality Management System (QMS)
- Automate document control with versioning, e-signatures, and audit trails to ensure GxP compliance.
- Implement Quality by Design principles with real-time tracking of deviations, CAPAs, and SOPs.
- Maintain an inspection-ready environment with automated compliance checks for FDA, EMA, and other regulatory requirements.
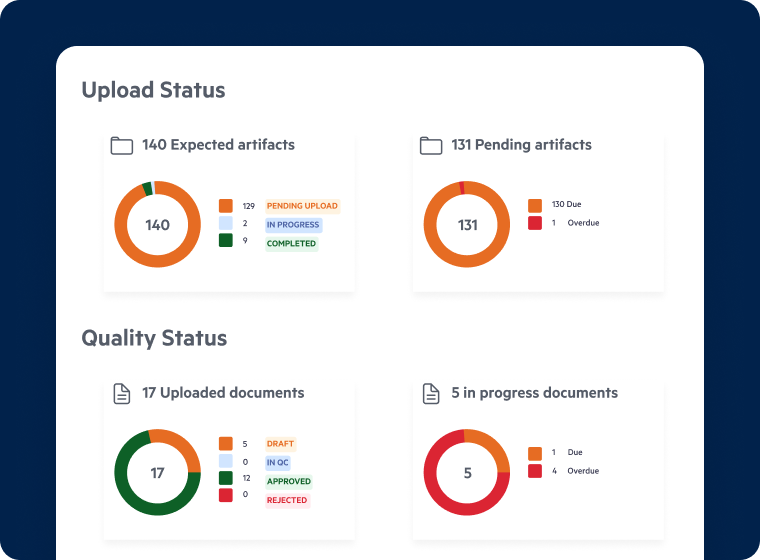
Secure and Efficient Regulatory Submissions
- Streamline submission workflows with automated metadata tagging and regulatory templates.
- Ensure seamless collaboration between internal teams, CROs, and regulatory agencies with secure document sharing.
- Improve submission accuracy and reduce errors with AI-powered document review and validation.
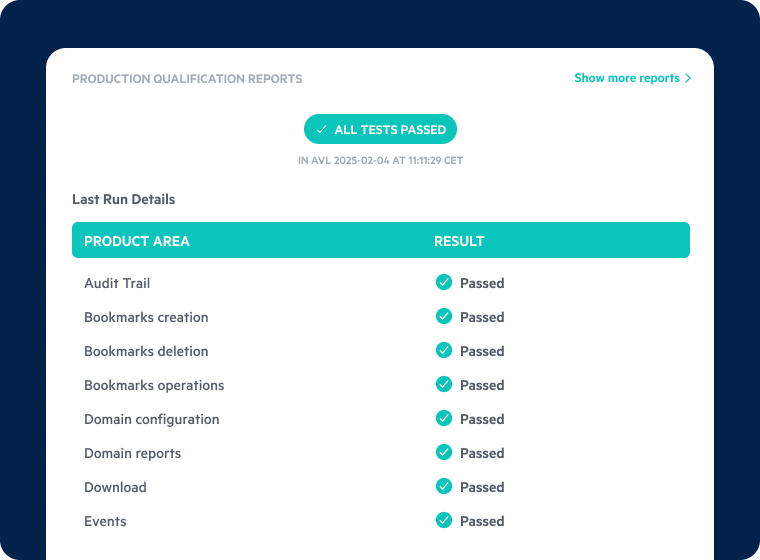
Inspection-Ready Audit Trails and Data Governance
- Maintain end-to-end traceability with automated compliance monitoring and detailed audit logs.
- Protect intellectual property and patient data with granular access controls and encryption.
- Support ALCOA+ principles with intelligent metadata management for accurate, consistent documentation.
Integration With Leading
Life Sciences Applications
Egnyte integrates seamlessly with your systems, making it easy to manage, collaborate on, and maintain compliance for your clinical files.






